Pippali (Piper longum) Part 2
Pippali
(Piper longum) Part 2
Modern
View
Piperine
Molecular
formula: C17H19NO3
Structural
formula:
Piperine was discovered and
isolated in 1819 by Hans Christian Orsted from fruits of black and white
pepper. Piperine is also found in Pippali (Piper
longum). [65]
Piperine was first synthesized
in 1882. It is an alkaloid. The solubility of piperine in water is 40mg/L.
Piperine enhances the bioavailability of vitamins, minerals and curcumin. The
mechanism of this activity is still under study. Piperine is known to inhibit
the enzymes P-glycoprotein and cytochrome P450 3A4 (abbreviated CYP3A4) in
humans. Animal studies also show that piperine inhibits cytochrome P450 (CYP
450) enzymes that metabolize many drugs.
Piperine raises levels of
dopamine and serotonin. It induces natural sleep and serenity. It enhances
memory and recall. It relieves stress. It improves metabolism and helps weight
loss. By correcting malfunctioning of immune system, it improves immunity.
One may take piperine to
increase effect of nutrients such as vitamins, curcumin, beta-carotene and
selenium. Piperine can also be useful in respiratory distress, indigestion,
joint discomfort and low mood.
Piperine can interact with many
medications. It may have anti-platelet aggregation effect. It may cause GI
bleeding when taken at higher than suggested doses. [66], [67]
Piperine shows
anti-inflammatory, antioxidant, immunomodulatory, antibacterial, antiviral,
anthelmintic, antiparasitic, antihistaminic, antiasthmatic (brocncodilator),
anti-platelet aggregation, antihypertensive, hepatoprotective and antitumor
properties. [68]
A study in mice for Parkinson’s
disease showed that piperine exerted protective effect on dopaminergic neurons
via anti-inflammatory, antioxidant and anti-apoptotic properties. By these
mechanisms piperine could prevent and protect the animals from Parkinson’s
disease. This suggests that piperine may be used to treat Parkinson,s disease. [69]
By inhibiting tyrosine kinase,
piperine mitigates progression of hepatocellular carcinoma (HCC). Histological
study supported this observation. This effect was attributed to antioxidant and
free radical scavenging property of piperine. A study showed that piperine down
regulated expression of catalase in Hep G2 cells. Piperine ameliorated
diethylnitrosamine (DEN)-induced hepatocellular carcinoma (HCC) by inducing
apoptosis in an in vitro model.
Piperine targeted insulin-like-growth-factor-1 (IGFR 1),
fibroblast-growth-factor-receptor-1 (FGFR 1) and hepatocyte-growth-factor-receptor
(HGFR) or c-Met downstream pathways (also called tyrosine-protein kinase Met)
in Hep G2 cells.
Piperine is a potent inhibitor angiogenesis. This property
can be useful for the treatment of cancers. [70]
Piperine inhibits the growth of
human breast cancer cells without affecting the growth of normal mammary
epithelial cells.
Piperine inhibits cell cycle
progression and induces caspase-dependent apoptosis via the mitochondrial
pathway.
Piperine inhibits matrix
metalloproteinase-2 and 9 mRNA expression, as well as breast cancer cell
migration. [71]
Piperine inhibits the growth of
breast cancer xenografts in immune-deficient mice. [72]
Piperine inhibits the proliferation
of prostate cancer. However the apoptosis-inducing activity of piperine in these
cells is low. Piperine induces cell-cycle arrest by down regulating cyclin D1
and cyclin A. Piperine treatment induces autophagy in prostate cancer cells. [73],
Toxicity
of Piperne
Piperine is acutely toxic to
mice, rats and hamsters. The LD50 values for a single intravenous
(i. v.), intraperitoneal (i. p.), subcutaneous (s. c.), intragastric (i. g.)
and intramuscular (i. m.) administration to adult mice were 15.13, 43, 200, 330
and 400mg/kg bodyweight respectively. Most animals given a lethal dose died of
respiratory paralysis within 3-17 minutes. Histopathologic changes included
severe haemorrhagic necrosis and oedema in gastrointestinal tract, urinary
bladder and adrenal glands. [74]
Pipernonaline
Molecular
formula: C21H27NO3
Structural
formula:
Pipernonaline belongs to the
class of organic compounds known as benzodioxoles. Pipernonaline is a solid
insoluble in water. Within the cell it is located in the membrane. Pipernonaline
is found in many herbs and in spices. This makes pipernonaline a potential
biomarker for the consumption of many food products. [76]
Phytochemicals derived from the
fruit of Pippali (Piper longum) show
fungicidal activity against six pathogenic fungi: Pyricularia oryzae, Rhizoctonia
solani, Botritis cineria, Phytophthora infestans, Puccinia recondita and Erysiphe graminis. Of three
phytochemicals (Piperidine, Pipernonaline and Piperettine) piprenonaline
exerted the strongest fungicidal action.
[77]
The methanol extract of the
fruit of Pippali (Piper longum)
containing pipernonaline, was found to be larvicidal against larvae of Culex pipiens at a concentration of 10 μg /ml after 24 hours of exposure. This
activity was much higher than malathion, methyl chlorpyrifos and methyl pirimiphos
the three commonly used organophosphorus insecticides. [78]
A
crude methanol extract of the fruit of Pippali (Piper longum) containing pipernonaline showed a strong larvicidal
activity of 100% mortality of Aedes aegypti mosquito larvae. No such activity
was observed with phytochemicals piperine, piperlongumine and piperettine [79],
[80]
The ethanol-extract of the fruit of Pippali (Piper longum) shows appreciable antihyperlipidemic activity in vivo. This activity was attributed to
piperine, pipernonalin and piperlonguminine (C16H19NO3). The antihyperlipidemic
activity was comparable to simvastatin. [81]
Through production of reactive oxygen species (ROS) pipernonaline
exhibits apoptotic properties. This causes disruption of mitochondrial function
and induces apoptosis in prostate cancer cells. This was the first report of
use of pipernonaline on human prostate cancer. [82]
Piperettine
Molecular
formula: C19H21NO3
Structural
formula:
Piperettine belongs to the
class of organic compounds known as benzodioxoles. It is a yellow solid. It was
first identified and isolated from the extract of Mareechee (Piper nigrum) and later synthesized in
the laboratory. It is insoluble in water. Within the cell piperettine is
located in the cell membrane. Outside of human the body piperettine can be
found in many herbs and spices. This makes piperretine a potential biomarker for
the consumption of these food products. Its pharmacological actions are not
studied yet. [84], [85]
Asarinine
Molecular
formula: C20H18O6
Structural
formula:
Asarinine belongs to
phenylpropene family. Asarinine is a physiologically active compound exerting
actions on some systems of the body.
Asarinin/Asarinine is one of
the slimming dietary supplements. [87]
Pellitorine
Molecular
formula: C14H25NO
Structural
formula:
Synonym:
(2E,
4E)-N-(2-Methylpropyl)-2, 4-decadienamide [88]
Pellitorine is a natural
product (alkaloid) obtained from the roots of Pellitor (Anacyclus pyrethrum), Mareechee (Piper nigrum) and some other plants.
Pellitorine was isolated from
the roots of Mareechee (Piper nigrum).
It is a very stable toxic, crystalline solid. [89], [90]
Pellitorine modulates the
function of sensory neurons and induces tingling sensation (paresthesia). Pellitorine
is inhibitor of Acyl-CoA cholesterol acyl- transferase and α-glucosidase. Pellitorine
exhibits anti-inflammatory, antibacterial, antimycobacterial and larvicidal
properties. Pellitorine is used in research on inflammations, infections,
diabetes and cancers. [91], [92]
Piperundecalidine
Molecular
formula: C23H29NO3
Structural
formula:
Piperundecalinide
is an alkaloid. It belongs to the family of Phenylpropenes. It is found in
fruits of Pippali (Piper longum) and
many herbs. [94]
Piperundecalinide
belongs to the class of organic compounds known as benzodioxoles. Piperundecalinide
exists as a solid that is insoluble in water. Within the cell piperundecalinide
is located in the cell membrane. Outside the body piperundecalinide can be
found in herbs and spices. Piperundecalinide exhibits anti-inflammatory
activity and inhibits oxidative stress. [95]
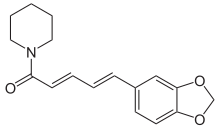
Piperlongumine
(PL)
Molecular
formula: C17H19NO5
Structural
formula:
Piperlongumine (PL) is a constituent of the fruit of the Pippali (Piper longum). Piperlongumine shows anti-inflammatory, antioxidant and antitumor properties. [96]
Recently piperlongumine and its
derivatives were synthesized. They all were found to show anti-inflammatory
activity. However of these derivatives piperlongumine was the most potent
anti-inflammatory agent. [97]
Fibroblast-like synoviocytes
(FLS) play a key role in the development of rheumatoid arthritis (RA). Myeloid
derived suppressor cells (MDSCs) can suppress T cell responses and play an
important role in the regulation of autoimmune arthritis. A study showed that
piperlongumine reduced the arthritis score and histopathologic lesions in
collagen-induced arthritis (CIA) in mice. Piperlongumine (PL) also reduced the
levels of serum anti-collagen II antibodies (anti C II), tumor necrosis factor-α (TNF-α), interleukin (IL)-1
β, interleukin (IL)-23 and interleukin
(IL)-17 in collagen-induced arthritis in mice. Additionally piperlongumine
(PL) reduced secretion of IL-1 β,
interleukin (IL)-23 and interleukin (IL)-17 by TNF-α-stimulated human
rheumatoid arthritis (RA) fibroblast-like synoviocytes (FLS). Piperlongumine
significantly inhibited the migration and invasion of TNF-α-stimulated human
rheumatoid arthritis (RA) fibroblast-like synoviocytes (FLS). These results
suggest that piperlongumine may be a candidate for the treatment of rheumatoid
arthritis (RA). [98]
Piperlongumine suppresses the
ability of proliferation, invasion and cell migration (formation of metastasis)
of oral cancer cells. It also increases the chemosensitivity and
radiosensitivity of oral cancers. Thus piperlongumine can be an effective
therapeutic agent for oral cancer. [99]
APR-246 is a novel anticancer
compound under trial for the treatment of refractory hematological malignancies
and prostate cancers. It is also tried for the treatment of head and neck
squamous cell carcinoma (HNSCC). Piperlongumine is found to synergize APR-246.
Administration of piperlongumine along with APR-246 significantly suppresses
glutathione S-transferase activity
resulting in the accumulation of reactive oxygen species (ROS), depletion of
glutathione (GSH) and elevation of oxidized glutathione (GSSG) in cancer cells.
This results in DNA damage of cancer cells and cancer cell apoptosis. [100]
A
study showed that piperlongumine inhibited the proliferation of all B-cell
acute lymphoblastic leukemia (B-ALL) but not normal B-cells. The effect was
dose and time dependent. Regardless of glucocorticoid resistance, piperlongumine
induced apoptosis in B-cell acute lymphoblastic leukemia (B-ALL) via elevation
of reactive oxygen species (ROS). Piperlongumine did not sensitize most of
B-cell acute lymphoblastic leukemia (B-ALL) to dexamethasone. [101]
Piperlongumine
inhibits Burkitt lymphoma but leaves normal cells undamaged. [102]
Piperlongumine
induces the DNA damage, apoptosis of breast cancer cells and inhibits the
proliferation of breast cancer cells. Piperlongumine also arrests the tumor
angiogenesis and formation of metastasis of breast cancer. [103]
Piperlongumine
was known to sensitize breast cancers to radiation. To validate this concept
the researchers cultured breast cancer MDA-MB-231 cells. The result of the
study showed that piperlongumine at a non-cytotoxic concentration enhanced the
radio-sensitivity of MDA-MB-231 cells. [104]
Piperlongumine
was found to be effective to arrest triple negative breast cancer. This
activity was attributed to the activation of mitochondrial apoptotic pathway.
The effect was dependent on the concentration of piperlongumine and duration of
the treatment. [105]
By raising intracellular levels of
reactive oxygen species (ROS) piperlongumine kills high-grade glioma (HGG)
without damaging normal brain cells. Therefore piperlongumine can be considered
a novel therapeutic option for the treatment of high-grade glioma (HGG). [106]
A study showed that piperlongumine selectively
killed glioblastoma multiforme cells via accumulating reactive oxygen species
in the cancer cells, leaving the normal cells unharmed. [107]
In an in vivo setting, piperlongumine
significantly reduced atherosclerotic plaque formation as well as proliferation
and activation of nuclear kappa B (NF-κB)
factor. [108]
At the
concentration of 100μg/ml
piperlongumine caused 30% inhibition of platelet aggregation. At the
concentration of 200μg/ml piperlongumine inhibited the platelet aggregation
induced by arachidonic acid (100%), collagen (59%), adenosine diphospate (ADP
52%) but not induced by thrombin. Acetyl salicylic acid (aspirin) a well-known
cyclooxygenase inhibitor greatly increased the effect of piperlongumine on
arachidonic acid-induced platelet aggregation.
[109]
In yet another study on rabbits,
piperlongumine, depending on concentration, significantly inhibited platelet
aggregation induced by thromboxane (A2) receptor agonist U46619 but only
slightly inhibited the platelet aggregation induced by thrombin. [110]
Piperlongumine suppressed the
growth of lung cancer cells. Most of 2-halogenated piperlongumine showed more
potent antitumor activity than the natural compound as it suppressed the tumor
growth by 48.58 percent at a dose of 2mg/kg body weight. This activity was
attributed to the ability of these compounds to elevate reactive oxygen species
(ROS) in tumor cells. [111]
Researchers showed that piperlongumine
inhibited the growth of colon cancer cells in time and concentration dependent
manner. At concentration below 10 μM
(micrometer) piperlongumine was not toxic to normal colon-mucosa cells. Even
after exposure up to 24 hours piperlongumine was not toxic to normal cells of
the colon-mucosa. [112]
By activating AMPK phosphorylation piperlongumine
shows cytotoxic activity against cultured HepG2 cells. [113]
Pancreatic cancer is one of the
most deadly cancers with 95% mortality. The poor response to currently
available therapies has drawn attention to alternative therapeutic strategies.
The use of reactive oxygen species (ROS)-inducing agents has emerged as an innovative
and effective strategy for the treatment of various cancers. By elevating
reactive oxygen species (ROS) and causing DNA damage, piperlongumine induces
apoptosis in pancreatic cancer cells. [114]
In
an experimental study, lupus-prone MRL-Fas (Ipr) female mice were treated by
intraperitoneal injection of piperlongumine at a dose of 2.4mg/kg bodyweight
for 10 days. The treatment significantly attenuated proteinuria and
glomerulonephritis. The improvement was accompanied by decreased serum levels of
nephritogenic anti-dsDNA antibodies, interleukin (IL)-6, interleukin (IL)-17, interleukin
(IL)-23 and TNF- α.
This study sheds new light on the immune-modulatory role of
piperolongumine. [115]
In
prostate cancer cell line elevated NF- κB activity had been demonstrated. This
was found in androgen-independent prostate cancers with metastasis.
Piperlongumine inhibited NF- κB activity and aggressive growth of prostate cancer cells.
Piperlongumine also inhibited the formation of metastasis of prostate cancers.
[116]
Androgen
receptor (AR) signaling is regarded as the driving force in prostate
carcinogenesis. Recent investigations show that piperlongumine induces rapid
depletion of the androgen receptor in prostate cancer cells. This study suggests
that piperlongumine may offer opportunities for both prevention and treatment
of prostate cancer. [117]
A study showed that
piperlongumine selectively inhibited the growth of human ovarian cancer cells.
Piperlongumine induced apoptosis in ovarian cancer cells. In vertebrates the
DNA replication occurs during synthesis (S) phase (G2 Phase or Gap 2 phase)
resulting in cell growth. Piperlongumine arrested the cell growth of ovarian
cancer cells in G2 phase that resulted in cell death. Combination of piperlongumine
with cisplatin or paclitaxel had synergistic antigrowth effect on ovarian
cancer cells. [118]
Piperlongumine is selectively
toxic to cancer cells in vitro and in vivo. By inducing oxidative stress in
cancer cells, causing DNA damage and apoptosis, piperlongumine kills cancer
cells. [119], [120]
Piperlongumine blocks the
nuclear factor κB (NF-
κB) pathway in inflammation activated
by tumor
necrosis factor-α (TNF-α)
and various other cancer promoters. Additionally piperlongumine also
shows activity against COX-2 and interleukin-6. Thus by potent
anti-inflammatory activity piperlongumine exhibits anticancer property. [121]
Retrofractamide-A
Molecular
formula: C20H25NO3
Structural
formula:
Retrofractamide
A belongs to the class of organic compounds known as benzodioxoles.
Retrofractamide A is a neutral solid that is insoluble in water.
Retrofractamide A is soluble in chloroform, benzene, ether, methanol and
ethanol. Within the cell Retrofractamide A is present in the cell membrane.
Outside of the human body retrofractamide A can be found in herbs and spices
such as black pepper. [122]
Pergumidiene
Molecular
formula: C27H39NO3
Structural
formula:
Pergumidiene was first isolated
from Marichi (Piper nigrum). Later it
was shown to be present in many piper species such as Piper longum, Piper cubeba.
Pergumidiene showed antibacterial activity against Staphylococcus aureus, Streptococcus
mutans and antifungal activity against Candida
albicans and Saccharomyces cerevisiae.
[124]
Brachystamide-B
Molecular
formula: C26H37NO3
Structural
formula:
Pharmacological properties of
Brachystamide B are similar to other phytochemicals of Pippali (Piper longum) [125]
Longamide
Molecular
formula: C7H6Br2N2O2
Structural
formula:
Longamide belongs to the class
of organic compounds known as carboxidimic acids. Longamide exists as a solid
insoluble in water. Within the cells longamide is located in the cell membrane.
Outside the human body longamide can be found in many herbs and spices such as
Pippali (Piper longum), Marichi (Piper nigram). [126]
Longamide shows cytotoxic
activity. Longamide is used to treat adenocarcinoma of the lung and human
prostate cancer. [127]
Dehydropipernonaline
Molecular
formula: C21H25NO3
Structural
formula:
Dehydropipernonaline, an amide
was isolated from Pippali (Piper longum)
Dehydropipernonaline has coronary vasodilating activity. [129]
Piperidine
Molecular
formula: (CH2)5NH
Structural
formula:
Piperidine is a colourless,
water miscible liquid. Its odor is described as objectionable or that of
typical amines or animal like. The name comes from Piper which is the Latin word for pepper.
Piperidine was first obtained
in 1850 by the Scottish chemist Thomas Anderson and later in 1852 by the French
chemist Auguste Cahours who named it ‘piperidine’. Both chemists obtained
piperidine by reacting piperine with nitric acid.
Piperidine and its derivatives
are used in the synthesis for new drugs and chemicals:
Icaridin
(Insect repellent)
Selective
serotonin reuptake inhibitors (SSRIs)
Neurostimulents
and nootropics:
Methylphenidate,
Ethylphenidate, Pipradrol, Desoxypipradrol
Opiods:
Dipipanone, Fentanyl and its
analogs, Loperamide, Pethedine, Prodine
Antipsychotic
medications:
Droperidol, Haloperidol,
Melperone, Mesoridazine, Risperidone, Thioridazine
Anticholinergic
chemicals and weapons:
Ditran, N-Methyl-3-piperidyl
benzilate
Selective
estrogen receptor modulators (SERM)
Raloxiphen
Vasodilators:
Minoxidil
Piperidine is also used in the manufacture of flavours and
fragrance agents.
Piperidine is also used in chemical degradation reactions
such as the sequencing of DNA in the cleavage of particular modified
nucleotides.
Piperidine is listed as a precursor of Narcotic Drugs and
Psychotropic Substances due to its use in the manufacture of ‘Angel Dust’,
‘Sherms’, ‘Wet’ etc. [PCP-(1-(1-phenylcyclohexyl)piperidine] [130]
Tetrahydropiperine
(THP).
Molecular
formula: C17H23NO3
Structural formula:
Tetrahydropiperine is an off-white solid with low melting point and
characteristic odor. Tetrahydropiperine is insoluble in water but soluble in
chloroform. Tetrahydropiperine is a natural topical permeation enhancer. Is
therefore used in cosmetic preparations for better penetration and to enhance
drug absorption.
Tetrahydropiperine can be used to treat febrile convulsions, epilepsy
and to relieve pain.
In agriculture Tetrahydropiperine is used to control insects. [132]
Sarmentine
Molecular formula: C14H23NO
Structural formula:
Sarmentine is a natural amide isolated from the fruit of Piper species. It is a potent herbicide.
Hence it is used more in agriculture. Sarmentine has some structural similarity
to crotonoyl-Co A, the substrate of enoyl-ACP reductase, a key enzyme in the
early steps of fatty acid stnthesis. This activity has still remained
unattended by researchers for its use in clinical medicine. [134]
References
[65]
https://en.wikipedia.org/wiki/piperine
[66]
GK Parish-Philip, 6 Impactful Benefits of Bioperine and Piperine Supplements,
March 2, 2018
[68]
Bhawna Chopra et al, Piperine and its Various Physicochemical and Biological
aspects: A Review, Open Chemistry Journal, Volume 5 , 2018
[69]
Wei Yang et al, Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,
2, 3, 6-tetrahydropyridine-induced Parkinson’s mouse model, International
Journal of Molecular Medicine 36: 1369-1376, 2015
[70]
Vetrichelvi Gunasekaran et al, Targeting hepatocellular carcinoma with piperine
by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo
study, Food and Chemical Toxicology, Volume 105, July 2017, Pages 106-118
[71]
Carolyn D. Doucette and Ashley L. Hilchie, Piperine a dietary Phytochemical, inhibits
angiogenesis, The Journal of Nutritional Biochemistry, Volume 24, Issue 1,
January 2013, Pages 231-239
[72]
Anna L.Greenshields and Carolyn D. Doucette, Piperine inhibits the growth and
motility of triple-negative breast cancer cells, Cancer Letters, Volume 357
Issue 1 February 2015, Pages 129-140
[73]
Dong-yunOuyang, Piperine inhibits the proliferation of human prostate cells via
induction of cell cycle arrest and autophagy, Food and Chemical Toxicology,
Volume 60, October 2013, Pages 424-430
[74]Pawinee
Piyachaturawat et al, Acute and subacute toxicity of piperine in mice, rats and
hamsters, Toxicology Letters,Volume 16, Issue 3-4, May 1983, Pages 351-359
[76]
http://www.hmdb.ca/metabolites/HMMDB0030339
[77]
Sung-EunLee, Fungicidal activity of pipernonaline, a piperidine alkaloid
derived from long pepper, Piper longum L., against pathogenic fungi, Crop Protection,
Volume 20, Issue 6, July 2001, Pages 523-528
[78] Lee SE, Mosquiti larvicidal
activity of pipernonaline, a piperidine alkaloid derived from long pepper,
Piper longum, J Am Mosq Control Assoc. 2000 Sep; 16 (3): 245-247
[79]
Yang YC et al, A piperidine amide extracted from Piper longum L. fruit shows
activity against Aedes aegypti mosquito larve, J Agri Food Chem. 2002 Jun 19;
50 (13): 3765-3767
[80]
Chaithong U et al, Larvicidal effect of pepper plants on Aedes aegypti (L.), J
Vector Ecol. 2006 Jun; 31 (1): 138-144
[81]
Jin Z et al, Antihyperlipidemic compounds from the fruit of Piper longum L,
Phytother Res. 2009 Aug; 23 (8): 1194-1196
[82]
Wan Lee, Pipernonaline from Piper longum
Linn. induces ROS-mediated apoptosis in human prostate cancer PC-3 cells,
Biochemical and Biophysical research Communications, Volume 430, Issue 1, 4
January 2013, Pages 406-412
[84]
F. S. Spring and James Stark, Piperettine from Piper nigrum, its isolation,
identification and synthesis, Journal of the Chemical Society, 1965,
[88]
https://pubchem.ncbi.nlm.gov/compound/Pellitorin#section
[89]
Oxford Dictionary
[90]
https://www.biovision.com/pellitorine.html
[91]
https://www.scbt.com/scbt/product/pellitorine-18836-52-57
[92]
https://www.biovision.com/pellitorine.html
[96]
https://en.wikipedia.org/wiki/Piperlongumine
[97]
Young Hwa Seo et al, Synthesis and biological evaluation of piperlongumine
derivatives as potent anti-inflammatory agents, Bioorganic and Medicinal
Chemistry Letters 2014 December 15, 24 (24): 5727-5730
[98] Jian Sun et al, Piperlongumine
attenuates collagen-induced arthritis via expansion of myeloid-derived
suppressor cells and inhibition of the activation of fibroblast-like
synoviocytes, Molecular Medicine Reports 2015, 11 (4): 2689-94
[99]
Yin-Ju Chen, Piperlongumine inhibits cancer stem cell properties and regulates
multiple malignant phenotypes in oral cancer, Spandios Publication, Published
on line on November 24, 2017, Pages: 1789-1798
[100]
Wei Hang, Piperlongumine and p53-reactivator APR-246 selectively induce cell
death in HNSCC by targeting GSTP1, Oncogene Volume 37, Pages 3384-3398, 18
January 2018
[101] Seong-Su Han et al,
Piperlongumine inhibits the proliferation and survival of B-cell acute
lymphoblastic leukemia cell lines irrespective of glucocorticoid resistance,
Biochemical and Biophysical Research Communications 2014, September 26, 452
(3): 669-75
[102] Seong-Su Han et al,
Piperlongumine inhibits proliferation and survival of Burkitt lymphomain vitro,
Leukemia Research 2013, 37 (2): 146-54
[103] U Bharadwaj et al,
Drug-repositionig screening identified piperlongumine as a direct STAT3
inhibitor with potent activity against breast cancer, Oncogene 2015 March 12,
34 (11): 1341-53
[104] Jian-Xin Yao et al,
Radio-sensitization by piperlongumine of human breast adenoma MDA-MB-231 cells
in vitro, Asian Pacific Journal of Cancer Prevention, 2014, 15(7): 3211-7
[105] Shweta Shrivastava et al,
Piperlongumine, an alkaloid causes inhibition of PI3 K/Akt/m TOR signaling axis
to induce caspase-dependent apoptosis in human triple-negative breast cancer
cells, Apoptosis: An International Journal on Programmed Cell Death, 2014, 19
(7): 1148-64
[106]
Tae Hyong Kim et al, Piperlongumine treatment inactivates peroxiredoxin 4,
exacerbates endoplasmic reticulum stress and preferentially kills high grade
glioma cells, Neuro-onchology, 2014, 16 (10): 1354-64
[107]
Ju Mei Liu et al, Piperlongumine selectively kills glioblastoma multiforme
cells via reactive oxygen species accumulation dependent JNK and p38
activation, Biochemical and Biophysical Research Communications 2013 July 19,
437(1): 87-93
[108] Dong Ju Son et al, Piperlongumine inhibits
atherosclerotic plaque formation and vascular smooth muscle proliferation by
suppressing PDGF receptor signaling, Biochemical and Biophysical Research
Communications 2012 October 19,
427 (2): 349-54
[109] Juvenia B
Fontenele et al, Antiplatelet effects of piplartine, an alkamide isolated from
Piper tuberculatum: possible involvement of cyclooxigenase blockde and
antioxidant activity, Journal of Pharmacy and Pharmacology 2009, 61(4): 511-5
[110] Masaya Iwashita
et al, Piperlongumine, a constituent of Piper longum L., inhibits rabbit
platelet aggregation as thromboxane (A2) receptor antagonist, European Journal
of Pharmacology 2007 September 10, 570 (1-3): 38-42
[111]
Yuelin Wu et al, Design, synthesis and biological activity of piperlongumine
derivatives as selective anticancer agents, European Journal of Medicinal
Chemistry 2014 July 23, 82: 545-551
[112] H Randhawa et al, Activation of
ERK signaling and induction of colon cancer cell death by piperlongumine,
Toxicology in Vitro: An International Journal Published in Association with
BIBRA 2013, 27 (6): 1626-33
[113]
Jahee Ryu et al, Piperlongumine as a potential activator of AMP-activated
protein kinase in HepG2 cells, Natural Product Research 2014, 28 (22):2040-3
[114]
Harsharan Dhillon et al, Piperlongumine induces pancreatic cancer cell death by
enhancing reactive oxygen species and DNA damage, Toxicology reports, 2014, 1:
309-318
[115] Lan Yao et al, Piperlongumine
alleviates lupus nephritis in MRL-Fas(Ipr) mice by regulating the frequency of
Th 17 and regulatory T cells, Immunology Letters 2014, 161 (1): 76-80
[116] Serge Ginzburg et al,
Piperlongumine inhibits NF- κB activity and attenuates aggressive growth
characteristics of prostate cancer cells, Prostate 2014, 74 (2):177-86
[117] Konstantin V Golovine et al,
Piperlongumine induces rapid depletion of the androgen receptor in human
prostate cancer cells, Prostate 2013, 73 (1): 23-30
[118]
Li-Hua Gong et al, Piperlongumine induces apoptosis and synergizes with
cisplatin and paclitaxel in human ovarian cancer cells, Oxidative Medicine and
Cellular Longevity 2014, 2014: 906804
[119]
Bosc E et al, Piperlongumine and some of its analogs inhibit selectively the
human immunoproteosome over the constitutive proteasome, Biochem Biophys Res
Commun 2018 Feb 12: 496 (3): 961-966
[120]
Daniel P. Bezera et al, Overview of the therapeutic potential of piplartine
(piperlongumine), European Journal of Pharmaceutical Sciences, Volume 48, Issue
3, 14 February 2013, Pages 453-463
[121]
Jia Gang Han et al, Piperlongumine
chemosensitizes tumor cells through interaction with cysteine 179 I κB
α
kinase, leading to suppression of NF-κB-regulated gene products, Molecular
Cancer Therapeutics 2014, 13(10): 2422-35
[122]
http://www.hmdb.ca/metabolites/HMDB0033447
[124]
Kamal Raj Aneja et al, Antibacterial efficacy of fruit extracts of two Piper
species against selected bacteria and oral fungi pathogens, Braz J Oral Sci.
Oct/Dec 2010-Volume 9, Number 4
[125]
https://pubchem.ncbi.nlm.nih.gov/compound/10047263#section=Top
[126]
http://www.hmdb.ca/metabolites/HMDBoo38842
[127]
Deng-GaoZhao et al, Total synthesis and cytotoxic activities of longamide B,
longamide B methyl ester, hanishin and their analogues, Bioorganic and
Medicinal Chemistry Letters, Volume 26, Issue 1, 1 January 2016, Pages 6-8
[128]
https://pubchem.ncbinlm.nih.gov/compound/Dehydropipernonaline
[129]
Shoji N et al, Dehydropipernonaline, an amide possessing coronary vasodilating
activity, isolated from Piper longum L, J Pharm Sci 1986 Dec; 75(12):
1188-9
[134]
Franck E. et al, Sermantine, a natural herbicide from Piper species with multiple herbicide mechanisms of action, Front
Plant Sci. 2015; 6: 222














Comments