Some important phytochemicals from Yashtimadhu-Licorice (Glycyrrhiza glabra)
Some important phytochemicals from
Yashtimadhu-Licorice
(Glycyrrhiza glabra)
By
Dr.
Hemant Vinze (M. S.)
Introduction
Yashtimadhu-licorice (Glycyrrhiza glabra) is used mainly for the treatment of
pharyngitis, laryngitis, respiratory diseases such as bronchitis; GI conditions
such as gastritis (hyperacidity) and in many polyherbal medicinal formulations.
Yashtimadhu-licorice (Glycyrrhiza glabra),
in fact, exhibits pleotropic pharmacological activity. Needless to say this is
due to many pharmacologically active phytochemicals contained in it. In this
article I present a few important pharmacologically active phytochemicals from Yashtimadhu-licorice
(Glycyrrhiza glabra).
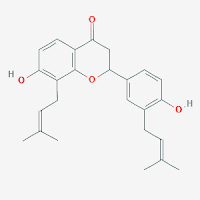
Glabrol
Molecular formula: C25H28O4
Structural formula:
Glabrol
is an acyl-coenzyme A. It is isolated from the roots of yashtimadhu-licorice (Glycyrrhiza glabra). Cholesterol acyltransferase
esterifies free cholesterol in the liver and the intestine. Accumulation of
these esters produce atherosclerosis. By inhibiting cholesterol
acyltransferase glabrol prevents the development of hypercholesterolemia
and atherosclerosis. Therefore glabrol can be used for the prevention and
treatment of obesity and type 2 diabetes and atherosclerosis. [1], [2]
By
binding to gamma aminobutyric acid (GABA) receptors glabrol exhibit hypnotic
effect. When administered at 5, 10, 25 and 50 mg/kg body weight glabrol
increased the duration of sleep and decreased sleep latency in a dose-dependent
manner. The hypnotic effect of glabrol was blocked by flumazepil, a
selective benzodiazepine receptor antagonist. [3]
Glabrolide:
Molecular formula: C30H44O4
Structural formula:
Glabrolide
was isolated from yashtimadhu-licorice (Glycyrrhiza
glabra) by Shen et al.
223-acetoxyglycyrrhetic
acid is the parent compound of glabrolide. The ' trivial' name (that is the
name not recognized according to the rules of any formal system of chemical
nomenclature) "glycyrrhetol" was used in the English abstracts for
glabrolide.
Glabrolide
belongs to 'triterpenoids' group of chemicals.
Glabrolide
is practically insoluble in water and weak acidic solvents.
Glabrolide
is found in many herbs and spices. Isolated in pure form glabrolide does not
actively exert pharmacological action but acts as a catalyst to other phytochemicals
found in yashtimadhu-licorice (Glycyrrhiza
glabra).
Glabrolide
can be found in yashtimadhu-licorice (Glycyrrhiza
glabra), many herbs and spices. Glabrolide is a biomarker for the
consumption of these food products. [4], [5], [6]
Isoglabrolide:
Molecular formula: C30H44O4
Structural formula:
Isoglabrolide
is a member of the class of compounds known as triterpenoids. Isoglabrolide
isinsoluble in water and weak acidic solvents. It is found in
yashtimadhu-licorice (Glycyrrhiza glabra),
many herbs, tea and spices. Isoglabrolide is a biomarker for consumption of
these food products. [7], [8]
Glycyrrhetol
Molecular formula: C30H48O3
Structural formula:
Glycyrrhetol
is a member compounds known as triterpenoids. Glycyrrhetol is insoluble in
water and weak acidic solvents. It is found in yashtimadhu-licorice
(Glycyrrhiza glabra), tea and spices. Glycyrrhetol is a biomarker
for consumption of these food products. [9]
Flavones/Flavonoids
The flavonoid
constituents in yashtimadhu-licorice (Glycyrrhiza glabra) mainly include
flavones, flavonals, isoflavones, chalcones, bihydroflavones and
bihydrochalcones. They show antioxidant, antibacterial, antiviral, antitumor
activities.
They
inhibit HIV infection. [10]
Till
1994, eight saponins, seven flavonoid glycosides and eleven flavonoids had been
isolated from licorice. Later five new flavonoid compounds isolated from
yashtimadhu-licorice (Glycyrrhiza glabra)
were: glucoliquitrin apioside,
phenyllicoflavone A (a flavonone bisdesmoside), shinflavone
(a bisflavone), shinpterocarpin (a phenylated pyranoflavonone), and
1-methoxyphaseollin, (both pyranopterocarpans). [11]
A
new flavonoid compound isolated from yashtimadhu-licorice (Glycyrrhiza glabra) named licoagrodione was found to show antimicrobial
activity indicated by disc diffusion method. [12]
Triterpenoids/ Triterpenes
Triterpenes
are a class of chemical compounds composed of three terpene units with the
molecular formula C30H48. These may be broadly divided according to the number
of rings present.
The
triterpenoids isolated recently from yashtimadhu-licorice (Glycyrrhiza glabra) exhibited a broad spectrum anti-viral
activity.[13]
Isoliquiritigenin
Molecular formula: C15H12O4
Structural formula:
Isoliquiritigenin
is a phenolic compound found in yashtimadhu-licorice (Glycyrrhiza glabra).
The
enzyme isoliquitrigenin 2-O-methyltransferase further transforms
isoliquitrigenin into 2-O-methylisoliquiritigenin.
Isoliquiritigenin
is a potent benzodiapin receptor modulator.
Isoliquiritigenin
is under trial for the treatment for cocaine addiction and various cancers.
[14]
Isoliquiritigenin
exhibits anti-inflammatory, anti-oxidant, immunoregulatory, anti-microbial,
hepatoprotective, cardioprotective and anticancer activities. However further
studies are needed to verify the target organ toxicity. [15]
A
study showed that isoliquiritigenin inhibited antral growth and altered
estradiol, testosterone and progesterone levels. These data indicate that
exposure to isoliquiritigenin inhibits growth and disrupts steroid production
in antral follicles. [16]
Isoliquiritin
Molecular formula: C21H22O9
Structural formula:
Isoliquiritin
is a member of the class of compounds known as flavonoid-o-glycosides.
Isoliquiritin is practically insoluble in water and weakly acidic
solvents. Isoliquiritin can be found in yashtimadhu-licorice (Glycyrrhiza glabra), fruits and tea.
This makes isoliquiritin a potential biomarker for the consumption of these
food products. [17], [18]
Isoliquiritin
flavonoid possesses anti-inflammatory, anti-oxidant, immunoregulatory and
antidepressant properties. The mechanisms of neuroprotective and antidepressant
activities are however unclear. The results of some studies showed that
pretreatment of PC12 cells with isoliqutritin significantly prevented
corticosterone-induced cell apoptosis, increased the activity of
superoxidedismutase (SOD) and catalase (CAT), decreased the contents of
reactive oxygen species (ROS) and malondialdehyde (MDA). Isoliquiritin thus
provides protective action against cortisone-induced cell damage by reducing
oxidative stress. Pretreatment with isolquiritin prevented cortisone-induced
mitochondrial damage. Isoliquritin attenuated intra-cellular calcium [Ca]
overload. Thus isoliquitrin exhibits cytoprotective and neuroprotective
effects. [19]
Liquid
extract of yashtimadhu-licorice (Glycyrrhiza
glabra) containing isoliquiritin inhibited granuloma angiogenesis.
Isoliquiritin thus exhibits antiangiogenic effect. Needless to say the effect
is dose dependent. [20]
Isoliquiritin
extracted from root of yashtimadhu-licorice (Glycyrrhiza glabra) and its derivatives displayed hypoglycemic
effects in Swiss albino male mice. [21]
Isoliquiritin
apioside from yashtimadhu-liquorice (Glycyrrhiza
glabra) shows a marked potential to combat oxidative stress- induced
genotoxicity. [22]
Licochalcone A
Molecular formula: C21H22O4
Structural formula:
Licochalcone
A is a chalconoid, a type of natural phenols. It was first identified in the
root and rhizome of yashtimadhu-licorice (Glycyrrhiza
glabra). It shows pleotropic pharmacological actions. [23]
Licochalcone
A displays a strong anti-inflammatory activity. Licochalcone A could
be an anti-inflammatory drug for future. [24]
Acne
is inflammation of sebaceous glands. Moisturizers and anti-inflammatory agents
are useful in controlling this condition. A study shows that the combination of
licochalcone A, L-carnitine and 1, 2-decanediol is useful in this regard. In
this formulation, licochalcone A has anti-inflammatory
effect, L-carnitine decreases sebum production and 1, 2-decanediol
has anti-bacterial effect. [25]
Licochalcone
A a major ingredient of yashtimadhu-licorice (Glycyrrhiza glabra) effectively inhibits sUV-induced COX-2 expression
and prostaglandin E2 PGE2 generation through the inhibition of activator
protein 1 (AP-1) transcriptional activity.
This protects the skin from UV radiation. Researchers feel Licochalcone A
may have potential for development as a skin cancer chemopreventive agent. [26]
Licochalcone
A also displays antibacterial, antiviral (especially against influenza
nuraminidase) and antimalarial activities. Its anti-inflammatory activity is
useful as a skin lightening agent particularly for acne rosacea and atopic dermatitis.
[27]
Yashtimadhu-licorice
(Glycyrrhiza glabra) contains many
varieties of licochalcone such as licochalcone A B C etc. They display a
strong antioxidant activity [28]
By
virtue of its antioxidant property licochalcone A suppresses the proliferation
of certain cancer cells. [29]
Licochalcone
A showed a dose dependent muscle relaxant effect in carbachol (at effective
concentration 50%)-induced bladder contraction. Pretreatment with licochalcone
A enhanced the relaxant effect of an adenylyl catalase activator,
forskolin. [30]
Licochalcone
A isolated from Glycyrrhiza species
induces apoptosis in cancer cells via Sp 1 and Sp 1 regulatory proteins
oral squamous cell carcinoma [31]
A
study published in Cancer Letter shows that Licochalcone A inhibits the growth
of gastric cancer cells by arresting cell cycle progression and inducing
apoptosis. The study suggests that licochalcones can be used in future to treat
gastric cancers. [32]
Isoangustone A
Molecular formula: C25 H26O6
Structural formula:
Isoangustone
A is a novel flavonoid found many species of Glycyrrhiza and many herbs. Isoangustone A was
first isolated from the roots of Glycyrrhiza
uralensis (Chinese licorice). Isoangustone A was present in
hexane/ethanol extract of Glycyrrhiza ralensis. Isoangustone A
is predominantly known as antineoplastic agent as in a study it induced
apoptosis in human prostate cancer cells. [33]
Isoangustone
A has anti-inflammatory and anticancer effects. Isoangustone
A inhibits cell proliferation by targeting PI3K, MKK4 and MKK7 in human
melanoma. [34]
In
another study, Isoangustone A present in hexane/ethanol extract of Glycyrrhiza uralensis induced
apoptosis in human prostate cancer cells. [35]
Licuraside:
Molecular formula: C26H30O13
Structural formula:
Licuraside
is a flavonoid isolated from various Glycyrrhiza species.
It inhibits the effects of tyrosinase. It shows antibrowning and depigmenting
activity on the skin. It is therefore used in cosmetic creams to lighten the skin
color, protect the skin from sun burns. [36], [37]
Roasting
of species of Glycyrrhiza with
refined honey improves the bioavailability, pharmacological activities and properties
of water-soluble phytochemicals such as liquiritin, isoliquiritin, apioside,
licuraside and glycyrrhizin. After processing the properties of these
phytochemicals were found to have changed for better. According to Ayurveda honey
should not be roasted because on roasting honey loses its medicinal properties.
Here roasting of honey works as a catalyst. Honey does not take part in
pharmacological activity of these phytochemicals. On roasting glycyrrhizin and liquiritin are
decomposed. [38]
Kumatakenin
Molecular formula: C17H14O6
Structural formula:
Kumatakenin
is an O-methylated flavonol. Kumatakenin was first isolated from the
flower bud of clove (Syzygium
aromaticum). Kumatakenin exhibits significant cytotoxic activity against human
ovarian cancer cells, SKOV3 and A2780.
Kumatakenin
increases the activity of caspase 3, -8 and -9. Kumatakenin also reduces the
expressions of MCP-1 and RANTES which are major determinants of macrophage
recruitment at tumor sites in ovarian cancer cells. Moreover kumatakenin
inhibits the expression of M2 markers and cancer promoting factors, including
IL-10, MMP-2/-9 and VEGF. These studies suggest that kumatakenin may be
a promising drug for the treatment of ovarian cancer. [39]. [40]
Glyzarin
Molecular formula: C18H14O4
Structural formula:
Glyzarin
is a flavone isolated from yashtimadhu-licorice (Glycyrrhiza glabra) in 1977 by
Bhardwaj D. K., Seshadri T. R. and Singh R. Glyzarin is insoluble in water.
Glyzarin
exhibits chemical properties and pharmacological actions of flavones. [41],
[42]
References:
1. Choi JH et al, Inhibitory activity of
diacylglycerol aceyltransferase by glabrol isolated from the roots of licorice,
Arch Pharm Res. 2010 Feb; 33(2):237-42
2. Choi JH et al, Glabrol, an
aceytyl-coenzyme A: cholesterol aceyltransferase inhibitor from licorice
roots. J Ethnopharmacol 2007 Apr 4;
110(3):563-6
3. Suengmok Cho, Hypnotic effects and
GABA-ergic mechanism of licorice (Glycyrhhiza glabra) ethanol
extract and its major flavonoid constituent
glabrol, https://www.researchgate.net/publication/224867014, April 2012
4.
https://pubchem.ncbi.nlm.nih.gov/compound/90479675
5. http://www.hmdb.ca/metabolites/HMDB34515
6.
https://books.google.co.in/books?isbn=370916480X
7.
https://pubchem.ncbi.nlm.nih.gov/compound/15559941#section=Top
8.
http://www.hmdb.ca/metabolites/HMDB35886
9.
http://www.hmdb.ca/metabolites/HMDB39690
10. Xing GX et al, Advances in studies
on flavonoids of licorice, Zhongguo Zhong Yao Za Zhi, 2003 Jul;
28(7):593-7
11. Kitagawa I et al, Chemical studies
of Chinese licorice-roots.. I. Elucidation of five new flavonoid
constituents from the roots of Glycyrrhiza glabra L. collected in Xinjiang,
Chem Pharm Bull (Tokyo), 1994 May; 42(5): 1056-62
12. Li W et al, Antimicrobial flavonoids
from Glycyrrhiza glabra hairy root cultures, Planta Med. 1998 Dec; 64(8):
746-7
13. Pu JY et al, Antiviral research of
triterpenoids in licorice, Bing Du Xue Bao. 2013 Nov; 29(6): 673-9
15. Peng F et al, A Review: The
Pharmacology of Isoliquiritigenin. Phytother Res, 2015 Jul; 29(7):969-77
16. Mahalingam S et al, Effects of
isoliquiritigenin on ovarian antral follicle growth and steroidogenesis, Reprod
Toxico. 2016 Dec; 66:107-114
17.
https://pubchem.ncbi.nlm.nih.gov/compounds/Isoliquiritin
18. http://www.hmdb.ca/metabolites/HMDB37318
19. Zhou YZ et al, Protective effect of
isoliquitrin against corticosterone-induced neurotoxicity in PC 12 cells,
FoodFunct 2017 Mar 22; 8(3): 1235-1244
20. Kobayashi S et al, Inhibitory effect
of isoliquiritn, a compound in licorice root, on angiogenesis in vivo and
tube formation in vitro, Biol Pharm Bull, 1995; Oct; 18(10): 1382-6
21. Gaur R et al, In vivo anti-diabetic
activity of Isoliquiritigenin and liquiritigenin, Phytomedicine. 2014 Mar 15;
21(4):415-22
22. Evaluation of antigenotoxic activity
of isoliquiritin apisode from Glycyrrhiza glabra L. Toxicology in
vitro Volume 23, Issue 4, June 2009, Pages 680-
23.
https://en.wikipedia.org/wiki/Licochalacone_A
24. Qui Y et al, Anti-inflammatory
activity of licochalacone A isolated from Glycyrrhiza inflata, Z Naturforsch C
2008 May-June; 63 (5-6): 361-5
25.
https://clinicaltrials.gov/wiki/ct2/show/NCT022173054
26. Song NR et al, Licochalcone A, a
polyphenol present in licorice, suppresses UV-induced COX-2 expression by
targeting PI3K, MEK 1 and B0- Raf.
27.
https://en.wikipedia.org/wiki/Licochalcone_A
28. Franceschelli S et al,
Licochalcone-C extracted from Glucyrrhiza glabra inhibits lipopolysaccharide-
interferon-γ inflammation by improving antioxidant conditions and regulating
inducible nitric oxide synthase expression, Molecules , 2011 Jul 6 16 (7):
5720-34.
29. Chen X et al, Antioxidative
andanticancer properties of Licochalcone A from licorice, J Ethnopharmacol.
2017 Feb 23; 198: 331-337
30. Naqai H et al, Antispasmotic
activity of licochalcone A, a species-specific ingredient of Glycyrrhiza
inflata roots, J Pharmacol. 2007 Oct; 59(10): 1421-
31. Cho JJet al, Licochalcone A a
natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis
via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma, Int J
Oncol. 2014 Aug; 45(2): 667-74
32. Xiao XY et al, Licochalcone A
inhibits growth of gastric cancer cells by arresting cell cycle progression and
inducing apoptosis, Cancer Lett. 2011 Mar 1; 302(1): 69-75
33. https://pubchem.ncbi.nih.gov/compound/Isoangustone_A
34. Song NR et al, Isoangustone A, a
novel licorice compound, inhibits cell proliferation by targeting PI3K, MKK4
and MKK7 human melanoma, Cancer Prev Res (Phila). 2013 Dec; 6(12): 1293-303
35. Seon MR et al, Isoangustone A
present in hexane/ethanol extract of Glycyrrhiza uralensis induces apoptosis in
DU 145 human prostate cancer cells via the activation of DR4 and intrinsic
apoptosis pathway, Mol Nutr Food Res. 2010 Sep; 54(9: 1329-99.
36.
https://pubchem.ncbi.nlm.nih.gov/compound/6475724#section=Chemical-and-Physical-Properties
37. Fu B et al, Isolation and
identification of flavonoids in licorice and a study of their inhibitory
effects J Agric Food Chem. 2005 Sep 21; 53(19): 7408-14
38. Wang M et al, Influence of
honey-roasting on the main pharmacological activities and the water-soluble
active glycosides of licorice, Afr J Tradit. Complement Altern Med. 2011 Dec
29; 9(2):189-96. eCollection 2012.
39.
https://en.wikipedia.org/wiki/Kumatakenin
40. Woo JH et al, Effect of Kumatakenin
Isolated From Cloves on the Apoptosis of Cancer Cells and the Alternative Activation
of Tumor-Associated Macrophages, J Agric Foof Chem. 2017 Sep 13; 65(36):
7893-7899. Epub 2017 Sep 5.
41. Bhardwaj D. K., Seshadri T. R. and
Singh R. Glyzarin, a new isoflavone from Glycyrrhiza glabra (1977)
http://agris.fao.org/agris-search/search.do?recordID=GB19780226336
42.
http://www.hmdb.ca/metabolites/HMDB295334










Comments